Synchron, After years of focused on everything AI, the most talked-about topics in medical technology today are not robots and algorithms but rather the uniquely non-artificial capabilities of the human brain.
The FDA has just issued both a “leapfrog” recommendation and a ground-breaking approval for these brain-computer interfaces, a sci-fi-like family of technologies that scan brainwaves to operate external devices.
Synchron is hoping to be the next company to receive a historic approval from the agency. An implant that converts thoughts into motions on smartphones and tablets is being developed by a New York City-based startup with the goal of regaining communication for patients who have severe paralysis.
Thanks to a recently completed series B fundraising round that netted Synchron $40 million, its Stentrode technology will soon be tested in a clinical trial in the U.S.
The FDA could grant the gadget a first-of-its-kind approval if the experiment is successful; according to CEO Thomas Oxley, barring any regulatory hiccups, the device could be available “within three to five years.”
Since Synchron’s device directly straddles the line where technology and medicine converge, Oxley said that certification would have significant ramifications for both industries.
He told Fierce Medtech, “We’re developing technology that brings electronics into the brain without the need for open-brain surgery.” It opens up a route for direct brain control of gadgets, which hasn’t been conceivable before. As a result, individuals with paralysis, which was once thought to be an incurable ailment, may benefit from this technology.
The FDA is already aware of Synchron’s system because it was granted breakthrough device classification in August of last year, giving it a faster route through the FDA review process.
FDA Synchron series khosla venturesparkfiercebiotech authorizes a wireless brace that enhances hand function in stroke patients by utilising brainwaves
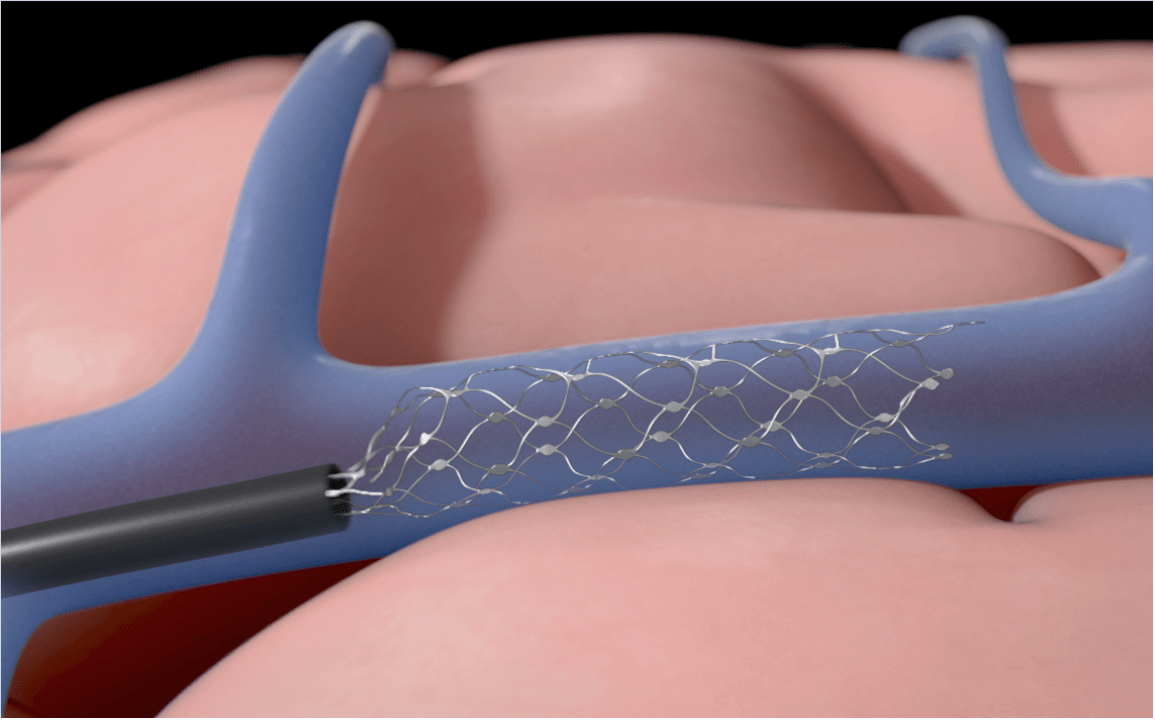
Three parts make up the technology, which is centered on the Stentrode implant itself. The system is the only brain-computer interface implant available today that doesn’t require open brain surgery. It is inserted into the jugular vein and extends to fit along the blood vessel’s walls while using built-in sensors to detect brain impulses.
According to Oxley, this positioning is what makes Synchron’s platform so ground-breaking.
“You can reach the deep and surface portions of the brain thanks to the blood arteries. Unlike other technologies that need open brain surgery, which necessitates the removal of the skull from every single part of the brain that needs to be accessed, “He clarified.
The Stentrode’s signals are transmitted to a receiver implanted in the patient’s chest. The data is then transferred from the battery-free BrainPort unit to Synchron’s BrainOS platform over Bluetooth, which may be downloaded to a user’s current smartphone, tablet, or computer. The technology enables users to translate the gathered brain signals into actions on messaging, email, banking, and retail apps.
In Australia, Synchron has already begun testing the system on people. Four study participants have received the Stentrode implant and have received training in using their thoughts to control a mouse to click or zoom in on a webpage. With the aid of a different eye movement tracker, the cursor is controlled.
The first two patients, who were both diagnosed with amyotrophic lateral sclerosis, demonstrated autonomous computer control with at least 92% accuracy in mouse clicks and an average typing speed of between 14 and 20 characters per minute, according to preliminary findings.
Synchron begins a clinical trial for a brain-computer interface without piercing patients’ skulls
Though the U.S. study will be the main priority, Synchron said it will also use some of the funding to advance the Stentrode system’s advancement.
The system might someday be utilised in the reverse manner, delivering signals to the brain to cure neurological diseases including Parkinson’s disease, epilepsy, depression, addiction, and more, in addition to using brainwaves to control equipment.
While Synchron’s technology is undoubtedly innovative, this revolution is not entirely new. According to Oxley of Fierce Medtech, a similar shift from mechanical to electronic technology occurred in cardiology in the 1990s, providing Synchron (and the rest of the world) with a roadmap for the future.
There is a template for how the sector needs to mature, he said, noting that we have a history of how it worked with cardiology.
Khosla Ventures, which recently invested in Docbot, Bionaut Labs, and another neurotech startup, Flow Neuroscience, lead the funding round. The funding more than quadruples Synchron’s prior round, a $10 million series A that includes involvement from the Defense Advanced Research Projects Agency of the U.S. Department of Defense.